Introduction
The adenovirus is a non-enveloped icosahedral virus whose genome is a linear, 36-Kb double-stranded DNA (dsDNA) molecule containing inverted terminal repeats (ITRs) at either end of the genome. We can divide its genes into early (E1-4) and late (L1-5) transcripts. Its transcription occurs in early and late phases before or after virus DNA replication, respectively. This involves a complex series of splicing events, which include four early regions of gene transcription (E1–E4) required for viral replication and an MLP that produces the late genes (L1–L5), generating the viral capsid.
Adenoviruses vectors
Adenoviruses infect a wide range of vertebrates, with over 50 serotypes in humans causing illnesses that range from mild respiratory infections to severe multi-organ diseases, commonly affecting the upper respiratory tract and leading to conditions like the common cold, conjunctivitis, and tonsillitis. Adenovirus has been widely studied as a gene transfer vector due to its well-understood genome, ease of genetic manipulation, broad host cell tropism, and ability to induce strong immune responses, making it a promising platform for vaccine development (Chang, 2021). Researchers most commonly use Ad5 among the 57 known human adenovirus types for vectors, utilizing CAR for cell entry.
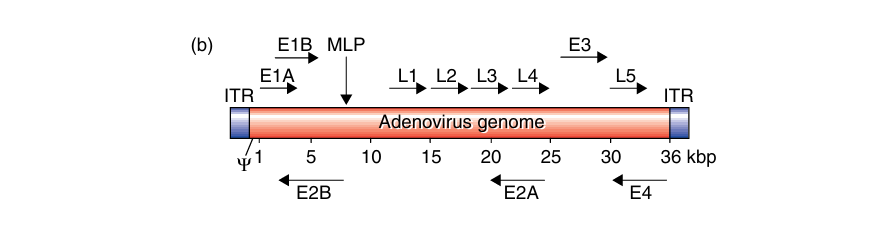
Adenoviral Components
Recombinant adenovirus vectors have the E1 and often the non-essential E3 gene deleted. This increases transgene packaging capacity to over 8 Kb, infecting both replicating and differentiated cells. The E3 gene: involved in evading host immunity and, thus not required for virus production. Some adenoviral vectors also lack E2 or E4 genes, further increasing foreign DNA capacity. E1 function is supplied in trans by packaging cell lines like 293 or 911, making the virus replication-deficient. The viral constructs include left and right arms to enable homologous recombination of the transgene into the adenoviral plasmid.
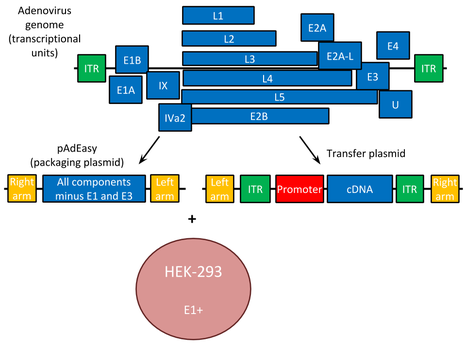
Transient gene expression occurs with adenoviral vectors because their DNA does not integrate into the host genome, thus they cannot bring about mutagenic effects caused by random integration events. It allows controlled, tissue-specific expression when foreign genes are regulated by cell-specific promoters and enhancers—an advantage in gene therapy trials
Adenovirus vectors generations
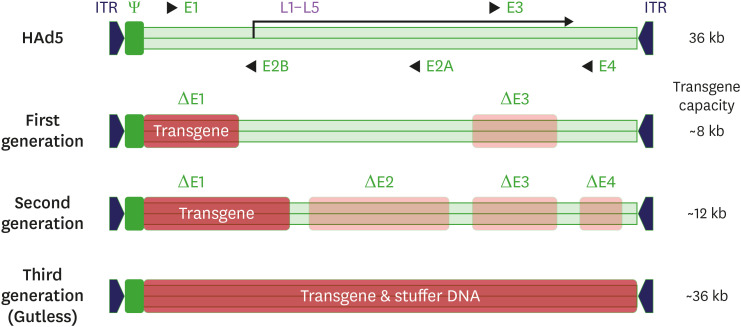
Adenoviral vectors have undergone significant genetic engineering across three generations to improve their safety and efficacy for human use.
- First-generation adenoviral vectors: created by deleting the E1 and E3 regions to insert a transgene and make the virus replication-defective, thus reducing its immunogenicity. However, these vectors may still produce replication-competent adenovirus (RCA) due to the homology between the vector genome and the E1 region in packaging cells, such as HEK293. Used cell lines like PERC.6, with reduced homology to minimize RCA formation.
- Second-generation adenoviral vectors further increase transgene capacity by deleting additional regions (E2/E4), which also lowers the risk of RCA formation. However, these modifications reduce the replication capacity of the vector, leading to lower production yields compared to first-generation vectors.
- Third-generation adenoviral vectors, also known as helper-dependent or gutless adenoviral vectors, remove nearly all viral genes except those necessary for packaging, such as inverted terminal repeats. These vectors can accommodate multiple transgene cassettes and have lower immunogenicity, but they are more complex to produce and carry the risk of helper virus contamination.
Adenovirus-Based Vaccine Development
Ebola and COVID-19 Vaccines
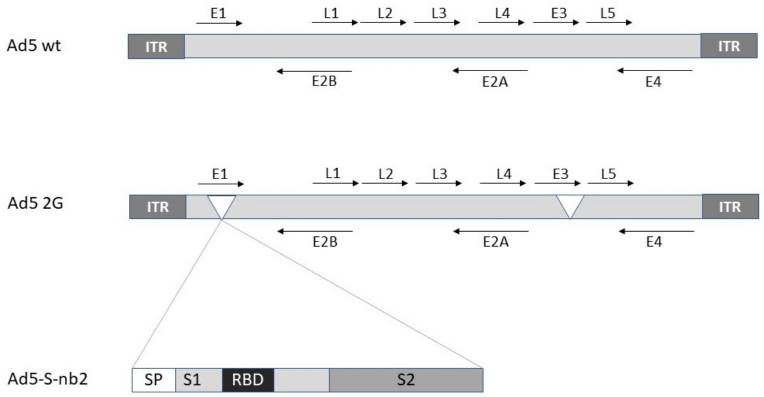
Adenovirus-based vaccines have a long history, with Ad26.ZEBOV being the first approved for human use in preventing Ebola, forming part of a two-dose regimen approved in Europe in 2020. Adenoviral vectors have also been key platforms in COVID-19 vaccine development, with candidates like HAd5, HAd26, and ChAdOx-1 (non-replicating vectors) undergoing large-scale trials. These vaccines often target the SARS-CoV-2 spike protein and can induce strong cellular and humoral immune responses. However, pre-existing immunity to certain adenoviruses (e.g., HAd5) can reduce vaccine efficacy, prompting the use of alternative vectors like ChAdOx-1, derived from chimpanzees, which showed safety and effectiveness in phase 2/3 trials.
Influenza Vaccine
Adenoviral vectors are being explored for universal influenza vaccines due to their ability to induce both humoral and cross-reactive T-cell immunity. Researchers target conserved viral components, like the HA stalk region or M2 ectodomain. HAd5-based influenza vaccines have shown promising safety and immunogenicity in trials. Current studies include developing an intranasal spray vaccine that could provide broad protection with long-lasting immunity.
HIV-1 Vaccine
Adenovirus vectors’ high immunogenicity made them promising for HIV-1 vaccines, but the HAd5-based STEP trial showed increased infection rates in HAd5-seropositive individuals, potentially due to CD4 T-cell activation. To overcome this, current research explores using different adenovirus serotypes for priming and boosting to avoid pre-existing immunity while maintaining strong immune responses.
Disadvantages and limitations of Adeno viral vectors (AVV)
The disadvantages of adenoviral vectors include the following:
- Expression is transient since the viral DNA does not integrate into the host.
- Adenoviral vectors are based on an extremely common human pathogen and in vivo delivery may be hampered by prior host immune response to one type of virus.
- Adenoviruses are highly immunogenic.
References
- Reece, R. J. (2004). Analysis of genes and genomes. Wiley.
- Chang, Jun. “Adenovirus Vectors: Excellent Tools for Vaccine Development.” Immune Network, vol. 21, no. 1, Jan. 2021, https://doi.org/10.4110/in.2021.21.e6.
- Addgene: Adenovirus Guide. www.addgene.org/guides/adenovirus.
- Lundstrom, Kenneth. “Viral Vectors for COVID-19 Vaccine Development.” Viruses, vol. 13, no. 2, Feb. 2021, p. 317. https://doi.org/10.3390/v13020317.


