The baculovirus, Autographa Californica (the alfalfa looper) multiple nuclear polyhedrosis virus (AcMNPV) has been used extensively as an expression vector using a genetically engineered cell line derived from the fall armyworm, Spodoptera frugiperda (SP9 and SP21).
Site-Specific Transposition of Target Genes into Baculovirus
Traditional methods for producing recombinant baculovirus vectors have two major drawbacks: (a) the time-consuming process of construction, plaque purification and confirmation of the structure of the desired recombinant virus can take 4-6 weeks, and (b) slow cell growth in expensive media, increasing contamination risks (Reece, 2004) and (Ciccarone et al, 2003). To address these issues, site-specific transposition of an expression cassette into a bacmid vector propagated in E. coli has been developed for high-level expression of recombinant proteins.
Construction of a Recombinant Bacmid
A bacmid is a baculovirus-plasmid hybrid molecule that functions as a shuttle vector, capable of replication in both E. coli and insect cells. Its construction involves the following key elements:
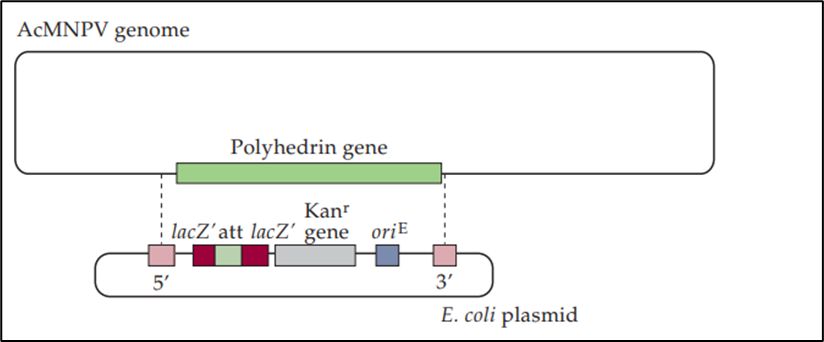
- An E. coli plasmid is incorporated into the AcMNPV (baculovirus Autographa Californica multiple nuclear polyhedrosis virus) genome by a double crossover event (dashed lines) between DNA segments (5′ and 3′) that flank the polyhedrin gene to create the bacmid.
- The E. coli plasmid DNA contains:
- the gene for resistance to kanamycin (Kanr) for selection of the bacmid in a plasmid;
- an attachment site (att) that is inserted into the LacZα gene without disrupting the reading frame of the 1acZa peptide;
- A low-copy number origin of replication (oriE) from F-plasmid for propagation in E. coli.
Site-Specific Transposition in Bacmid Construction
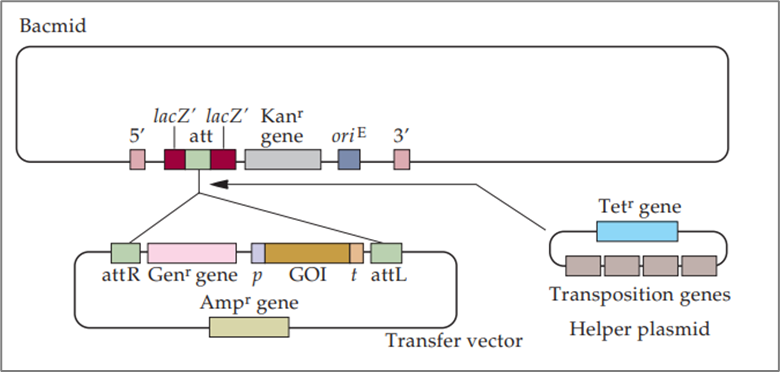
Site-specific transposition is a crucial step in the creation of recombinant bacmids. The recombinant bacmid can be isolated from E. coli and transfected directly into insect cells. This process involves three key components: the bacmid, a helper plasmid, and a transfer vector. Here’s how they interact:
- The transposition proteins encoded by genes of the helper plasmid facilitate the integration (transposition) of the DNA segment of the transfer vector that is bounded by two attachment sequences (attR and attL).
- The gene for resistance to gentamicin (Genr) and a gene of interest (GOI) that is under the control of the promoter (p) and transcription terminator (t) elements of the polyhedrin gene are inserted into the attachment site (att) of the bacmid.
- The helper plasmid and transfer vector carry the genes for resistance to tetracycline (Tetr) and ampicillin (Ampr), respectively.
The recombinant bacmid
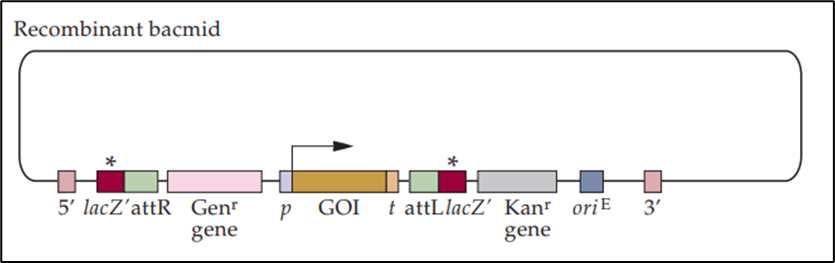
- The recombinant bacmid has a disrupted lacZ′ gene, indicated by (*), which occurs due to the insertion of the gene of interest and other elements during the transposition process.
- The right-angled arrow denotes the site of initiation of transcription of the cloned gene after transfection of the recombinant bacmid into an insect cell.
- Cells transfected with a recombinant bacmid are unable to produce functional Beta-galactosidase in the presence of Xgal and IPTG as a direct result of the disrupted lacZ′ gene.
The hybrid construct combines the advantages of bacterial plasmids with the insect cell infectivity of baculovirus, facilitating: easier genetic manipulation in bacterial systems and efficient protein expression in insect cells.
Advantages of Bacmid shuttle vectors
- Insect cells carry out post-translational processing of proteins similar to that of mammalian cells.
- In most cases, the protein of interest is highly expressed, soluble, and can be easily recovered from infected cells.
- The Bac-to-Bac system allows for the simultaneous production and isolation of multiple recombinant viruses.
- Baculoviruses are non-pathogenic to droids, mammals, and plants.
- Can accommodate large or multiple genes.
- Cell lines used to amplify the virus and express proteins grow well in suspension cultures allowing large-scale protein production in bioreactors.
- The system uses a variety of efficient gene promoters for early and late gene expression.
- Proper protein folding, glycosylation, phosphorylation, acetylation, and acylation
References
- Glick, B. R., Pasternak, J. J., & Patten, C. L. (2010). Molecular Biotechnology: Principles and Applications of Recombinant DNA. Amer Society for Microbiology.
- Reece, R. J. (2004). Analysis of genes and genomes. Wiley.
- Ciccarone, V. C., Polayes, D. A., & Luckow, V. A. (2003). Generation of Recombinant Baculovirus DNA in E. coli Using a Baculovirus Shuttle Vector. Humana Press eBooks, 213–236. https://doi.org/10.1385/0-89603-485-2:213


