Pichia pastoris overview
Pichia pastoris is a methylotrophic yeast capable of utilizing methanol as its sole energy and carbon source for growth. The first step in methanol metabolism involves the oxidation of methanol to formaldehyde by the enzyme alcohol oxidase (AOX1), using molecular oxygen (O₂) as a co-substrate. Alcohol oxidase (AOX1) has a low affinity for O₂, and to compensate, P. pastoris produces large amounts of the enzyme (Koutz et al., 1989). The AOX1 promoter, which regulates alcohol oxidase expression, is strongly inducible by methanol and tightly regulated, making it a powerful tool for heterologous protein expression (Tschopp et al., 1987).
Expression vectors containing target genes are typically integrated into the P. pastoris genome, either as single or multiple copies, enabling stable and consistent expression. For optimal protein production, P. pastoris cells are initially grown in glycerol, as glucose represses AOX1 transcription, even in the presence of methanol. Upon methanol induction, heterologous proteins can accumulate to high levels, facilitating efficient production of recombinant proteins in large quantities. The system’s ability to achieve high cell density cultivation and its strong inducibility under methanol make P. pastoris a valuable host for the industrial-scale production of recombinant proteins.
Protein expression in Pichia pastoris
Pichia has become an important host for recombinant protein expression because it offers high cell density, high yields, controllable processes, stability, and durability. Pichia also efficiently secretes heterologous proteins in defined media to save time and cost associated with purification. (source: pichia.com)
Pan et al. (2022) stated the following general advantages of protein production using P. pastoris system include higher folding efficiency, high cell density fermentation, strong expression system, genetic stability, and a mature secretion system of secreting proteins to the external environment.
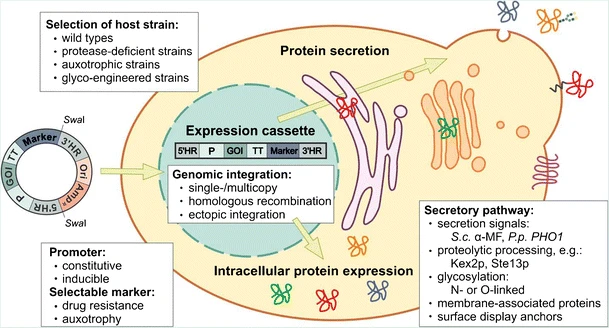
Practical Methods for Expression of Recombinant Protein in the Pichia pastoris System
Using P. pastoris as an expression system for heterologous proteins, Mohammadzadeh et al (2021) provided detailed basic protocols for cloning of a recombinant cassette into a suitable expression vector, the transformation of foreign vector DNAs into the yeast by electroporation, and expression and purification of desired recombinant protein:
- Cloning of a recombinant cassette into a suitable expression vector
- Transformation of P. pastoris and selection of transformants
- Optimization and large-scale expression of recombinant proteins
- Purification of recombinant proteins
Similarity to Saccharomyces
Many of the techniques developed for Saccharomyces may be applied to Pichia pastoris. These include:
- transformation by complementation,
- gene disruption,
- gene replacement
In addition, the genetic nomenclature used for Saccharomyces has been applied to Pichia. For example, histidinol dehydrogenase is encoded by the HIS4 gene in both Saccharomyces and Pichia. There is also cross-complementation between gene products in both Saccharomyces and Pichia. Several wild-type genes from Saccharomyces complement comparable mutant genes in Pichia pastoris. Genes such as ADE2, HIS4, LEU2, ARG4, TRP1, and URA3 all complement their respective mutant genes in Pichia. (PichiaPink™ Expression System User Guide).
Methanol free expression system
- The methanol induction system is widely used for its robust regulation and high-level recombinant protein expression in Pichia pastoris. However, the use of methanol presents safety concerns and requires strict process controls, particularly in large-scale fermentations. Efforts to develop methanol-free expression systems have emerged as alternatives.
- One such approach involved deleting three transcriptional repressors and overexpressing the transcription factor MIT1, which led to the successful construction of a methanol-free PAOX1 start-up strain (Wang J. J. et al., 2017).
- This innovation enables protein expression using a glucose-glycerol-shift induction model, replacing the conventional glycerol/methanol induction. While this system is safer, more economical, and environmentally friendly, it produces lower levels of protein expression (Wei et al., 2016).
Posttranslational modifications
Pichia pastoris offers significant advantages over Saccharomyces cerevisiae in glycosylation patterns, particularly for the production of therapeutic proteins. One key benefit is that Pichia is less prone to hyperglycosylation, producing shorter N-linked oligosaccharide chains (8–14 mannose residues) compared to S. cerevisiae (50–150 mannose residues) (Grinna & Tschopp, 1989; Tschopp et al., 1987b).
Additionally, Pichia avoids terminal α1,3 glycan linkages that are commonly present in S. cerevisiae, which are known to contribute to the hyper-antigenic properties of proteins, making them unsuitable for therapeutic applications. Proteins glycosylated in Pichia more closely resemble those in higher eukaryotes, making them more appropriate for biopharmaceutical development (Cregg et al., 1993; Gerngross, 2004; Hamilton et al., 2003; Hamilton & Gerngross, 2007).
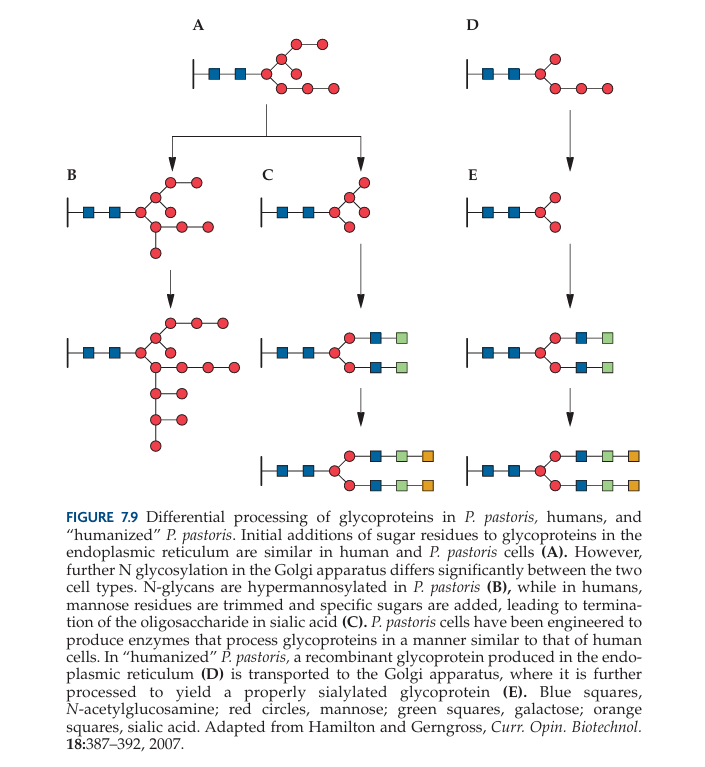
Protein expression between Pichia pastoris and Saccharomyces cerevisiae
| Feature | Pichia pastoris | Saccharomyces cerevisiae |
|---|---|---|
| Protein Yield | Higher protein yield, especially for secreted proteins | Moderate protein yield |
| Post-translational Modifications | Capable of performing eukaryotic modifications (e.g., glycosylation) but with shorter glycan chains | Performs eukaryotic modifications, but glycosylation patterns differ from humans |
| Secretion Efficiency | Efficient secretion system, ideal for expressing secreted proteins | Secretion can be less efficient |
| Induction System | Methanol-inducible system (e.g., PAOX1 promoter) or methanol-free options (glucose-glycerol-shift) | Uses constitutive and inducible promoters (e.g., GAL1) |
| Growth Rate | Slower growth compared to S. cerevisiae | Faster growth rate |
| Fermentation Scalability | Highly scalable for large-scale fermentation | Scalable, but may face limitations in industrial-scale settings |
| Cost of Culture | Lower cost, as P. pastoris can grow on cheaper carbon sources (e.g., methanol, glycerol) | Typically higher cost of media and culture maintenance |
| Protein Folding and Stability | Efficient folding and stability for complex proteins | Can sometimes struggle with folding of complex proteins |
| Glycosylation Hyper-Man | Produces less hypermannosylation, better for human-like glycosylation | Tends to hypermannosylate, problematic for some therapeutic proteins |
However, P. pastoris requires strictly-defined culture conditions, which may require more time and equipment than S. cerevisiae. S. cerevisiae has a wider range of resources and is supported by more literature
References
- Pan, Yingjie, et al. “Current Advances of Pichia Pastoris as Cell Factories for Production of Recombinant Proteins.” Frontiers in Microbiology, vol. 13, Nov. 2022, https://doi.org/10.3389/fmicb.2022.1059777.
- Mohammadzadeh, Roghayeh, et al. “Practical Methods for Expression of Recombinant Protein in the Pichia Pastoris System.” Current Protocols, vol. 1, no. 6, June 2021, https://doi.org/10.1002/cpz1.155.
- Iqbal, Omer MD. Molecular Biotechnology: Principles and Applications of Recombinant DNA, 4th Edition. Medicine & Science in Sports & Exercise 42(10):p 1972, October 2010. | DOI: 10.1249/MSS.0b013e3181e86fe5


